A New Test for SARS-CoV-2 Antibodies
Minimally invasive approach requires one drop of blood
Get all our news
By not requiring people to come into the clinic to have their blood drawn, we conserve clinical resources, keep people safe at home during shelter-in-place, and greatly increase the potential reach of antibody testing.”
Thomas McDade
Carlos Montezuma Professor of Anthropology and IPR Fellow
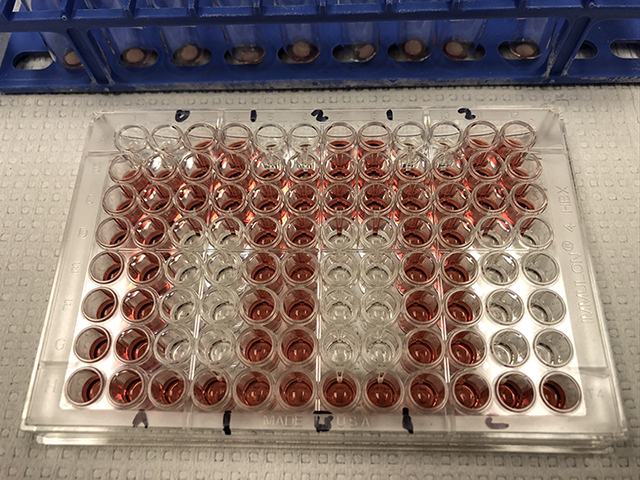
An assay plate contains samples of reconstituted blood collected from a finger prick that are ready for analysis.
As antibody testing ramps up across the country, a team of Northwestern University researchers have developed a new method for testing antibodies of SARS-CoV-2, the virus that causes COVID-19. The method requires only a single drop of blood collected from a simple finger prick.
The scientists reported this new test in a paper on MedRxiv, the preprint server for health sciences. IPR biological anthropologist Thomas McDade, the paper’s lead author, worked on the project with pharmacologist Alexis Demonbreun and investigators Richard D’Aquila, Brian Mustanski and Elizabeth McNally.
Antibody tests are useful for determining prior exposure to a virus, like the one that causes COVID-19. But current approaches to antibody testing have significant limitations: Point-of-care tests using finger-stick blood are qualitative and often inaccurate, while more precise lab-based tests require venous blood. The Northwestern team has developed an approach that combines the convenience of finger-stick blood sampling at home with the analytical rigor of a lab.
“As the first waves of infection subside, antibody testing is necessary for ascertaining the true prevalence and mortality rate of infection and for evaluating how effective policies such as social distancing or closing schools and restaurants are working to prevent viral transmission,” McDade said.

Currently, most viral testing has relied on polymerase chain reaction (PCR) analysis of nasal swabs to detect the virus’ presence. Serological testing, also known as antibody testing, serves a different and complementary function. It detects the presence of the Immunoglobulin M (IgM) and Immunoglobulin G (IgG) antibodies against SARS-CoV-2 in blood samples from previously exposed individuals.
Early in the pandemic, the research team realized there were important gaps in the current approach to antibody testing. They quickly assembled a unique team with complimentary expertise. Since testing materials—for all types of testing—have been in short supply, the team manufactured key reagents. Investigators worked in parallel on Northwestern’s Evanston and Chicago campuses to expedite development and ensure the test performed equally well in the hands of multiple researchers. The team next built a scalable test for broad community use.
According to McNally, “Widespread serological testing is essential for figuring out how the virus is spreading in the community, but it is very hard to screen large numbers of people when the tests require people to come to a healthcare provider.” She directs the Center for Genetic Medicine in the Feinberg School of Medicine.
The team’s testing approach builds on McDade’s pioneering work to develop field-friendly dried blood spot (DBS) methods in which drops of whole blood collected from a simple finger stick are stored on special filter paper, where it dries. He has developed DBS methods for multiple biomarkers of inflammation and immune function, and many of those methods have been incorporated into large, population-based studies over the past 15 years. This approach overcomes the logistical challenges associated with venous blood collection and facilitates the collection of blood samples outside of clinical settings at a low cost.
McDade said DBS sampling has several benefits that will promote more widespread screening.
“It is inexpensive, relatively painless, and people can collect their own blood and send samples to the lab through the regular mail,” McDade said. “By not requiring people to come into the clinic to have their blood drawn, we conserve clinical resources, keep people safe at home during shelter-in-place, and greatly increase the potential reach of antibody testing.”
The team’s future plans for testing are two-fold. “We are testing more samples so we can more formally evaluate the sensitivity and specificity of our antibody test,” McDade said. “Second, we are building a web-based platform to roll out antibody testing across Chicago. We are fortunate at Northwestern to have investigators like Brian Mustanski and Richard D’Aquila who have experience working with communities in Chicago studying viral infections.”
Other researchers who contributed to the Northwestern study include IPR research associate Aaron Miller; and Lauren Vaught, Nina Reiser, Elena Bogdanovic and Aaron Zelikovich, members of the Center for Genetic Medicine.
Funding from CIFAR (Canadian Institute for Advanced Research) and Northwestern supported the research.
For more information about the new test, see www.northwestern.edu/stories/.
Source: McDade, T., E. McNally, R. D’Aquila, B. Mustanski, A. Miller, L. Vaught, N. Reiser, E. Bogdanovic, A. Zelikovich, & A. Demonbreun. 2020. Enzyme immunoassay for SARS-COV-2 antibodies in dried blood spot samples: A minimally invasive approach to facilitate community- and population-based screening. MedRxiv preprint, MEDRXIV/2020/081844.
For more information about McDade's and other IPR faculty experts’ research, please visit IPR’s website and IPR’s COVID-19 research and media webpage.
Photo credits: T. McDade
Published: May 6, 2020.